The Rye Laboratory
Department of Biochemistry and Biophysics
Intracellular protein folding: GroELS
The intracellular protein folding problem.
Only part of the information needed to fold a protein into its functional three-dimensional or native (N) structure is encoded by the linear sequence of its amino acids. The remainder of this information comes from the specific environment in which the protein is located.
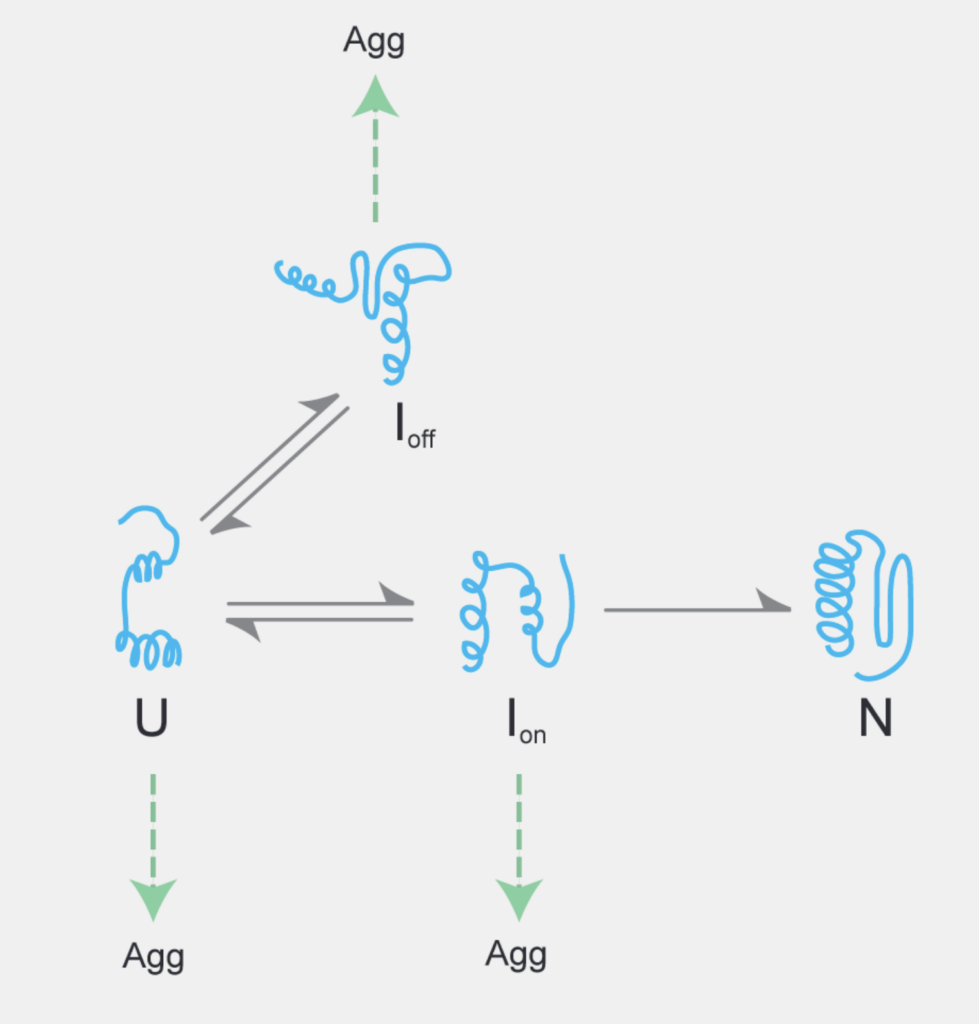
The concentrated and complex interior of a cell is an inherently hostile environment for the efficient folding of many unfolded (U) or partially folded (I) proteins. Once disentangled from an aggregate (Agg), or freshly synthesized from the ribosome, these proteins cannot fold autonomously to their native conformation.
Chaperonins are large, ATP-powered protein folding machines.
In the network of molecular chaperones that fold, monitor, and maintain cellular proteins, the large, barrel-shaped oligomers known as chaperonins play a central and essential role.
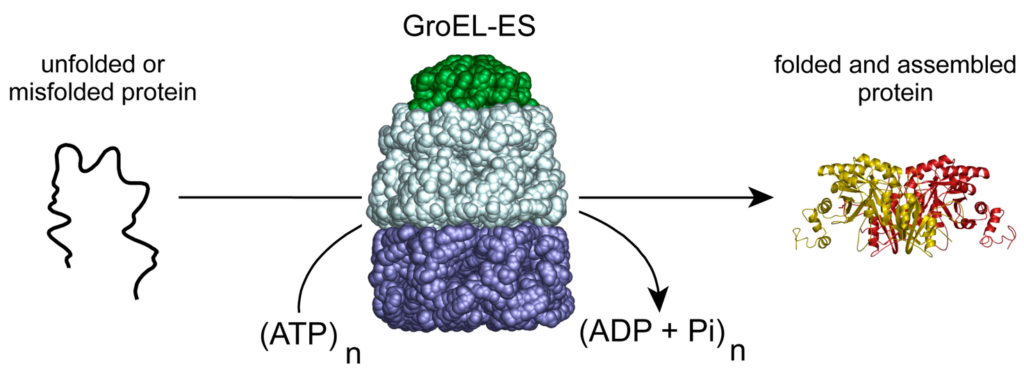
These remarkable molecular machines employ the energy of ATP hydrolysis to power a facilitated protein-folding reaction. GroELS, the chaperonin system of the bacterium Escherichia coli, is the archetypal member of this ubiquitous family of protein folding engines.
How does GroEL accelerate protein folding?
Exactly how chaperonins like the GroELS accelerate protein folding remains controversial. We are trying to unravel how this enhancement mechanism works. We employ a variety of biochemical and biophysical methods, including various types of fluorescence spectroscopy, rapid-mixing methods and single molecule techniques and we collaborate other groups bringing additional cutting-edge single molecule, mass spectrometry and cryo-electron microsocpy tools to bear on on this problem.
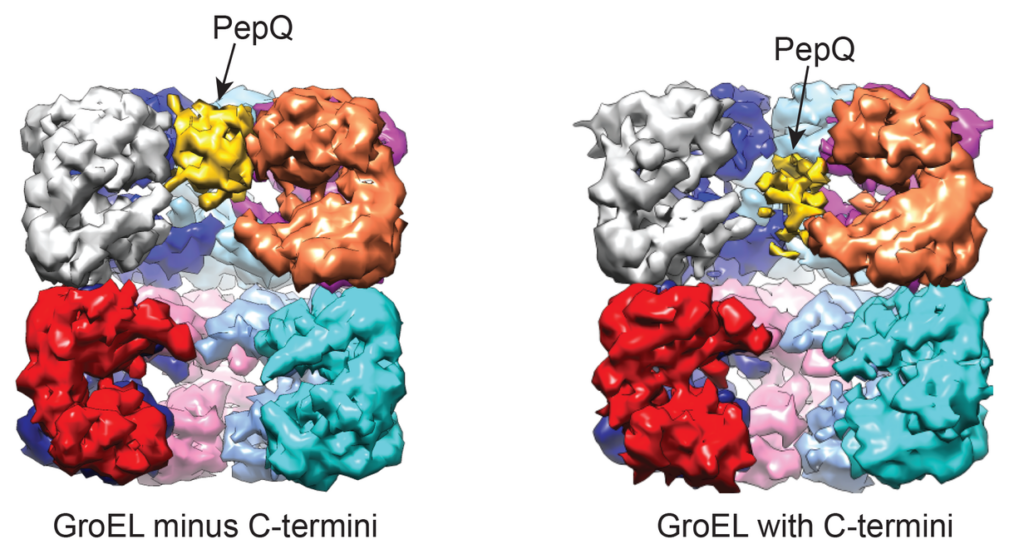
Above is the cryoEM structure of GroEL in complex with the non-native substrate protein, PepQ, which was solved in collaboration with the laboratory of Dr. Junjie Zhang at Texas A&M. When the C-terminal tails at the base of the GroEL cavity are intact, the PepQ protein is drawn toward the bottom of the cavity and its internal density decreases, consistent with a directed unfolding of the kinetically trapped PepQ folding intermediate. See Nat Commun. 8, 15934 (2017).