The Rye Laboratory
Department of Biochemistry and Biophysics
Secretory vesicle formation and dynamics
Creating a membrane-bound transport carrier.
The structural and metabolic stability of a eukaryotic cell depends on the trafficking of material between sub-cellular compartments. Intracellular trafficking requires the formation and correct targeting of small, membrane-bound vesicles or tubules.

These vesicular/tubular carriers (VTCs) are first created from a so-called donor compartment. Once fully formed, VTCs are then actively transported to, and subsequently fuse with, an acceptor compartment where they deliver their soluble cargo into the lumen of the acceptor compartment and release their membrane proteins into the acceptor compartment membrane.
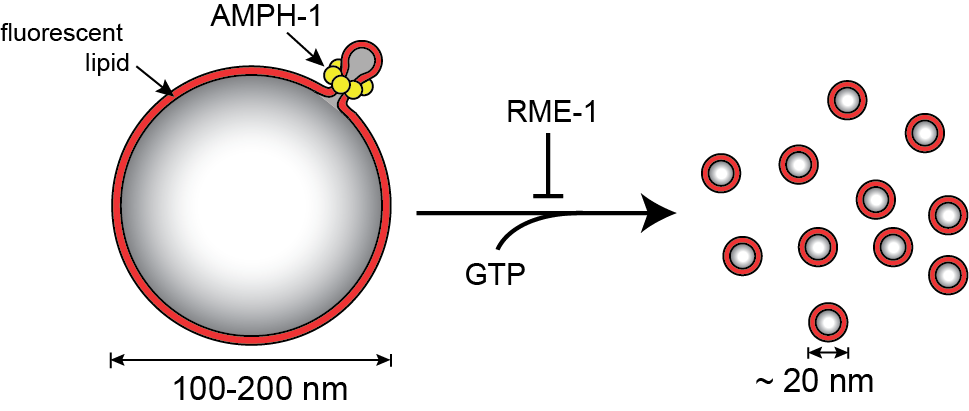
Membrane vesicle formation requires membrane fission.
While much is known about the protein coats that sculpt membranes into vesicles, the mechanism by which vesicles are released, the membrane fission reaction, remains poorly understood. We are using the release of transport carriers from the recycling endosome of C. elegans and from the plasma membrane of yeast as model systems for examining the mechanisms of membrane fission
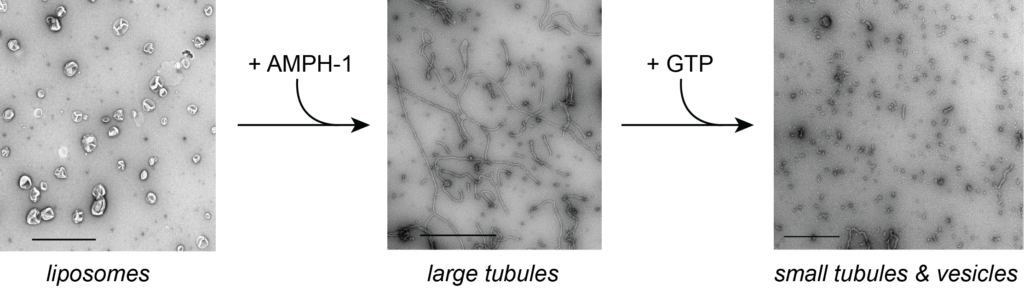
As models, we are focusing on the proteins AMPH-1 and RME-1 from worms and Rvs161/167p from yeast. Unexpectedly, we have found that the N-BAR proteins AMPH-1 and Rvs161/167 both display robust, GTP-stimulated membrane fission activity. See Traffic, 24: 34 (2023).
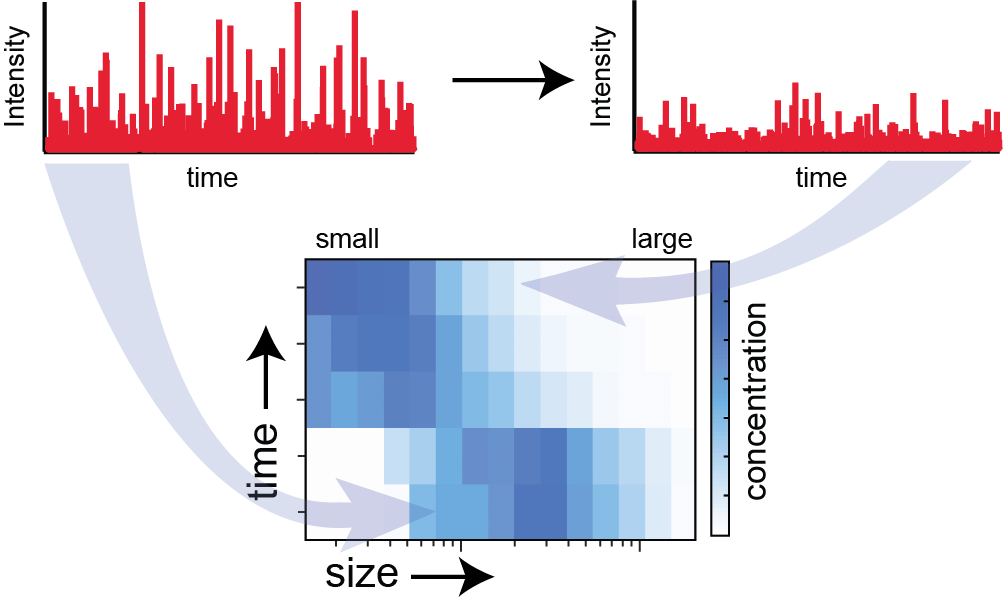
Real-time observation of membrane fission with BAS.
To try and understand the molecular details of this process, we are applying a variety of techniques, including whole cell fluorescence imaging, single molecule FRET, mass spectrometry and cryo-electron microscopy. We have also developed a cell-free, single particle fission assay based on BAS, which we are using to understand how nucleotide hydrolysis is coupled to membrane binding, rearrangement and fission.
Because BAS permits the real-time determination of complex particle distributions, sub-populations of fission intermediates, and their rates of interconversion, can be observed in a single sample. When fluorescent derivatives of fission proteins are employed, we can use MC-BAS to simultaneously observe the extent of protein binding to lipid objects of different sizes as fission takes place.